Tue 1st September 2020
CARBON (II) OXIDE
CARBON(II)OXIDE
Carbon(ii)oxide is poisonous colourless and odourless gas. It is present in coal gas and other gaseous fuels. It is produced by incomplete combustion of carbon compounds e.g Octane found in petrol.
Laboratory preparation of carbon(ii)oxide.
Carbon(ii)oxide is prepared in the laboratory by he action of concentrated tetraoxosulphate (vi)acid on methanoic acid or ethanedioic acid. This is a dehydrating reaction in which one molecule of water is removed.
Physical properties of carbon(ii)oxide.
- It is a colourlessodourlessandtastelessgas.
- It is neutral to litmus .
- It is slightly lighter than air.
- It is insoluble in water but dissloves in a solution of ammoniacal copper (I) chloride.
Tue 25th August 2020
CARBON(II)OXIDE
Carbon(ii)oxide is produced by incomplete combustion of carbon compounds like octane found in p
Tue, 11th August 2020
Good day students, how is everything over there? Hope you are preparing very well for resumption? May God almighty be with us all in Jesus name.
I have only one person that did my assignment,only Alayande Mercy, that is not good enough, remember that your note will be marked accordingly, so please endeavor to be up and doing.
Now let us go straight to the topic of today, we will be looking at Oxides of carbon today.
OXIDES OF CARBON
When carbon is heated in oxygen or air, it forms two types of oxides which are carbon(iv)oxide (CO2) and carbon(ii)oxide(CO). The oxide formed depends on the availability of oxygen. Let us take it one after the other.
CARBON(IV)OXIDE
The atmosphere contains about 0.03% by volume of carbon(iv)oxide, it doesn’t support combustion and it is very important to green plant.
Laboratory preparation of CO2.
Carbon(iv)oxide is prepared in the laboratory by the action of dilute hydrochloric acid or dilute trioxonitrate(v)acid on a trioxocarbonate (iv) or a hydrogen trioxocarbonate (iv). CaCO3+2HCl->CaCl2+H2O+CO2 OR. CaCO3+2HNO3->Ca(NO3)2+H2O+CO2. OR NaHCO3+HNO3->NaNO3+H2O+CO2.
Physical properties of CO2
- tis a colourless, odourless and tasteless gas.
- It is slightly soluble in water
- It is denser than air, about 1.95g/dm³.
- It is a weak acidic gas which turns moist blue litmus paper red.
- On cooling, it readily liquefies and solidifies at 78°c to form a white solid known as dry ice
Chemical properties of CO2
- Reaction with water:- CO2 react with water and dissolves to form trioxocarbonate (iv)acid which is a weak acid.
- Reaction with alkalis:-CO2 reacts with alkalis to produce trioxocarbonate (iv). CO2+2NaOH->Na2CO3+ H2O .
- Reaction with burning magnesium:- CO2 doesn’t support combustion but burning magnesium continues to burn in a gas jar of CO2 to produce carbon deposit and a white magnesium oxide ash. 2Mg+CO2-> 2MgO +C.
- Reaction with red-hot carbon:- when CO2 is passed over red-hot carbon, it is reduced to carbon(ii)oxide(CO) . CO2+C->2CO.
Test for carbon(iv)oxide
When CO2 is bubbled into limewater (calcium hydroxide) the limewater turns milky due to the precipitation of insoluble calcium trioxocarbonate (iv). Ca(OH)2+CO2->CaCO3+H2O.
Uses of CO2
1. it is used in fire extinguisher because it doesn’t support combustion.
2. It is used in the manufacture of mineral water like Coca-Cola, Pepsi, sprite etc because of it’s refreshing taste.
3. It is used as leavening agent in the baking of bread
4. Green plants use it during the process of photosynthesis.
5. It is used as a coolant in nuclear reactors .
ASIGNMENT
- State 4 uses of carbon(IV)oxide.
- State 4 physical and 4chemical properties of CO2.
- How can you test for CO2?
Tue 4th August 2020
Good morning students how was ur night? Hope u had a nice one?
Today we will be looking at the fuel gases that can be gotten from the products of carbon.
GASEFICATION OF COKE( fuel gases)
(A) Producer gas:- Producer gas is a mixture of carbon(ii)oxide and nitrogen, it is prepared by passing air over red-hot Coke in a furnace. The oxygen in the air oxidizes the coke to carbon (ii)oxide with the liberation of alot of heat while nitrogen is unchanged. Some carbon(iv)oxide formed are reduced by the hot coke to carbon (ii)oxide.
2C+O2+N2–> 2CO2+N2+heat (producer gas).
Uses of producer gas
- Producer gas is not expensive and it is used to heat furnace retorts during the manufacture of zinc and coal gas.
- It is also used to heat lime klins, steel and glass furnaces.
- It is a source of nitrogen for the manufacture of ammonia during Harber process.
Water gas:- Water gas is a mixture that contains equal volumes of carbon(ii)oxide and hydrogen. It is prepared by passing steam over white-hot Coke in a furnace at 1000°c.
C+H2O–>CO+H2+heat(water gas)
The production of producer gas is an exothermic reaction while the of water gas is endothermic reaction. Both are produced in the same plant known as d producer. The heat produced when producer gas is formed is sufficient for water gas formation.
Uses of water gas
- Water gas is more efficient fuel than producer gas because in water gas both hydrogen and carbon(ii)oxide burn in air releasing a lot of heat which makes water gas an important industrial fuel. The presence of carbon(ii) oxide makes it poisonous.
- Water gas is also used in the manufacture of hydrogen, methanol and butanol.
ASSIGNMENT
- Mention 2 gases responsible for the production of:-
(a) producer gas, (b) water gas.
(2) State 2 uses each of :-(a) producer gas. (b) water gas.
Tue 27th July 2020
Good day everyone how has your day been? I have the following names of students that did the assignment :- Alawode Fiyinfoluwa, Olawoye Tomiwa, Nwosu Kamsy, Ajibade Jumoke, Onyeaghala Dominion, Adekola Ibukun, Olatunji Sharon, Mercy Alayande, Jemimah Ademola and Omowaye Comfort.
Today we will breakdown the details about the products of the destructive distillation of coal.
COKE:- Coke is the non- volatile residue left behind after the destructive distillation of coal. It contains about 95% carbon and burn without smoke.
Uses:- (1) it is used as both industrial and domestic fuel. (2) it is used for the manufacture of gaseous fuel. (3) it is used as a reducing agent in the extraction of metals from their ores.
AMMONIACAL LIQUOR:- this is a solution of ammonia in water.
Use:- it is used in the preparation of ammonium tetraoxosulphate (vi) which is used as a fertilizer.
COAL TAR:- Coal tar is a thick black liquid which is a mixture of many organic substances and can be separated by fractional distillation.
Uses:- (1) Coal tar is used in the synthesis of some useful chemicals such as disinfectantants, explosives, drugs ,perfumes, dyes, synthetic fibres and solvents for rubber and paints.
COAL GAS :- Coal gas is a volatile component consisting of a mixture of gases such as hydrogen, methane, carbon(ii) oxide, ethene and small amount of impurities such as sulphur(iv) oxide and hydrogen sulphide.
Uses:- Coal is an important gaseous fuel because it is a cleaner and more efficient fuel than coal or other solid fuels.
ASIGNMENT
- State the products of the destructive distillation of coal.
- State 2 importance each of the above products.
Tue 20th July 2020
Good day everyone and how is everything over there? I noticed dt it is only 4 students answering my questions since , I want feedbacks from other people. Today we will be looking at the meaning of coal and the types.
COAL
Coal is a black organic rock formed from the vegetation of the Carboniferous period. It is gotten from the changes that took place from the remains of forest tress and vegetation buried underground millions of years ago under a great pressure and temperature in the absence of air.
TYPES OF COAL
There are 4 types of coal which are (i) peat coal (ii) lignite or brown coal (iii) bituminous or soft coal (iv) anthracite or hard coal. Going from peat to anthracite is called the rank of the coal.
Uses of coal
(I) Coal is used as fuel to generate power for steam engines , factories and electrical plants.
(ii) It is also used for domestic heating and for making various chemicals.
Destructive distillation of coal
When coal is heated at high temperature in the absence of air, it decomposes to give 4 main products which are Coke –> ammoniacal liquor–> coaltar –> coalgas . This process is known as destructive distillation of coal .
Assignment
- What do you understand by coal?
- State d types of coal
- Mention 2 importance of coal
- What are the products of the destructive distillation of coal?
Tue 14th July 2020
AMORPHOUS CARBON
Apart from coal which is mined from natural deposits, amorphous carbons are not considered as true Allotropes of carbon. Other amorphous carbons include :- (i) wood charcoal (ii) animal charcoal (iii) sugar charcoal (iv) carbon black, lamp black or soot.
(1) Wood charcoal:- Wood charcoal is prepared by burning wood in a limited supply of air.
Uses of wood charcoal
(i) it is used as domestic fuel
(ii) it is used for the purification of noble gases and the recovery of industrial solvent .
(iii) it is a good absorbent therefore it is used in gas masks for absorbing poisonous gases.
(2) Animal charcoal:- Animal charcoal is produced by heating bones in a limited supply of air.
Uses of animal charcoal
It has a property of absorbing colouring matter therefore it is used in decolorizing crude sugar and petroleum jelly.
(3) Sugar charcoal:- Sugar charcoal is formed when sugar is dehydrated either by burning the sugar in limited supply of air or by the action of concentrated tetraoxosulphate (vi) acid. C6H12O6 — concH2SO4—> 6C + 6H2O.
Uses of sugar charcoal
(i) it is used to prepare artificial diamond by heating strongly at high temperature.
(ii) it is used as a reducing agent in the extraction of metals.
(4) Carbon black, lamp black or soot :- These are produced by burning carbonaceous materials in a limited supply of air.
Uses
(i) carbon black is used as an additive to rubber in the manufacture of rubber tyres.
(ii) it is also used in making printer’s ink, carbon paper, black shoe polish, typewriting ribbons etc.
- What do you understand by amorphous carbons?
- state all the types of amorphous carbons.
- State 3 uses of wood charcoal.
The 7th July 2020
CARBON AND ITS COMPUNDS
Carbon is a non metal and it occurs naturally as diamond and graphite. Carbon also occurs in an impure form as coal and in a combined state as petroleum, wood and natural gases.
Allotropes of carbon
Allotropy:- it is the ability of carbon to exist in various forms in the same physical state.
Diamond and graphite are two allotropic forms of crystalline carbon. Others like coal, coke, charcoal, lampblack, sugar charcoal and animal charcoal are amorphous or non- crystalline form of carbon.
DIAMOND
Diamonds are purest form of naturally occurring carbon, it is a giant molecule in which the carbon atoms are closely packed and held together by strong covalent bonds.
Properties of diamond
(1) it is a colourless and lustreless solid which can be transformed into brilliant gems.
(2) it is octahedral in shape
(3) it has a high melting and boiling point
(4) it is a non- conductor of electricity because there are no free valence electrons in the diamond crystals.
(5) it is very dense and resistant to high temperature and chemical attack
(6) it has a density of 3.5g/cm³
USES OF DIAMOND
(1) Since diamonds are dense and hard, they are used industrially in drills for mining.
(2) it is used as abrasives to sharpen very hard tools and for cutting glass and metals.
(3) It is used as pivot support in precision instruments and as dies for drawing wires.
(4) it’s high refractive index and dispersion power make it useful as jewellery.
GRAPHITE
Graphite is formed by the action of volcanic heat on coal deposits. The carbon atoms in graphite form flat layers which are arranged in parallel one above the other to form a crystal lattice .
Properties of graphite
(1) Graphite is soft and flakes easily because of its layered crystalline structure.
(2)it has a hight melting point but less dense than diamond.
(3) graphite is a good conductor of electricity because of the mobile electron present in the crystal lattice.
(4) it has a density of 2.3g/cm³.
(5) graphite has hexagonal shape.
Uses of graphite
(1) graphite is used as dry lubricant due to its layered structure by allowing one layer to slide over another.
(2) it is non volatile and not sticky
(3) it is a good conductor of electricity
(4) a mixture of graphite and clay is used in making lead pencils.
(5)it is used as black pigment in paint and as a neutron moderator in atomic piles.
(6) since graphite can withstand high temperature, it is used to line crucibles used for making high grade steel and other alloys.
Assignment
- Define Allotropy
- State 2 crystalline allotropic forms of carbon
- State 3 uses each of diamond and graphite.
Tue 30th June 2020
WATER POLLUTION
Water pollution is making water unfit for the purpose that it is meant for. It is a big problem as it threatens aquatic life and changes water bodies into unsightly.
WATER POLLUTANTS
(1) Refuse and Sewage
(2) Industrial and agricultural waste
(3) Crudeoil spills
(4) Thermal pollution ( the use of water for cooling process)
(1) Refuse and Sewage:-Refuse or sewage is mostly organic matter, so when it is discharged into water bodies, it is broken down into some substances by the decomposers which are mainly bacteria, too much sewage in water body causes increase in the bacterial population which lead to the reduction in the level of oxygen in the water there by killing aquatic organism.
(2) Industrial and agricultural wastes:- Many factories empty their chemical wastes into the water bodies without converting them into harmless substances. The contamination results from the phosphate and nitrate present in the fertilizer which encourage the growth of large amount of algae . Many harmful chemical wastes like detergents and insecticide are non- biodegradable, i.e. they cannot be broken down into harmless compounds.
(3) Crude oil spills:-The spillage of oil floats on water and kills most of the marine life in the affected water, oil spills can also prevent people from using the water.
(4) Thermal pollution:- Many industries like oil refineries, steel Mills and breweries use water for cooling process,and when this warm water is released into water bodies, the increase in temperature of the water affects the aquatic life adversely.
CONTROL OF WATER POLLUTION
- Refuse should be buried or burnt in an incinerator to prevent air pollution.
- Strict laws must be passed to control water pollution
- Safety measures must be passed implemented to prevent crude oil spills.
- Sewage should be processed, treated and converted to useful fertilizers in Sewage plants.
- Chemical waste should be converted to harmless biodegradable substances before being dumped into the sea. ASSIGNMENT. 1. What do u understand by water pollution? 2. State 4 water pollutant. 3. State 4 control measures for water pollution.
Tue, 23rd June 2020
Good day students, hope u r coping with the weather? Always cover yourself up to avoid cold, take good care of yourself and stay safe.
We will look at the correction to the assignment through voice note.
PROPERTIES OF WATER
Physical properties of water
(1) pure water is a clear, colourless, tasteless and odourless liquid.
(2) It has a boiling point of 100 degree Celsius and a freezing point of 0 degree Celsius.
(3) It has a maximum density of 1gcm-³ at 4 degree Celsius
(4) It is neutral to litmus.
Chemical properties of water
(1) Reaction with metals:- Water reacts with metals to a degree varying with their position in the electrochemical series. So
(I) Potassium, Sodium and Calcium react with cold water to form alkalis and liberate hydrogen.
2K +2H2O — 2KOH + H2
2Na +2H2O — 2NaOH + H2
Ca + 2H2O — Ca(OH)2 + H2
(ii) Magnesium and zinc react with only steam to liberate hydrogen and form oxides. Mg + H2O — MgO +H2
(2) Reaction with non- metals :- Non-metals like carbon, chlorine and silicon also react with water.
Carbon reacts with steam at a white heat to form carbon(ii)oxide and hydrogen (water gas) C +H2O — CO + H2. Silicon react with steam at red-heat to form the oxide and liberate hydrogen. Si+H2O — SiO2 + 2H2
Tue, 16th June 2020
LESSON FIVE
Good day everyone, you are welcome to today’s class, how your days been? God is our strength in Jesus name. Let us look at the correction to the last assignment, it will be done in voice note.
WATER (CONTD)
PERMANENT HARDNESS OF WATER
Permanent hardness of water can only be removed by using chemicals. It is caused by the presence of calcium tetraoxosulphate (vi) and magnesium tetraoxosulphate (vi) in water.
REMOVAL OF PERMANENT HARDNESS.
(a) Distillation :- Both temporary and permanent hardness of water can be removed by distillation because all the ions present in the water are left behind after the water vapour distills. Distillation is rarely used because no Chemical is needed and it is not economical.
(b) Addition of washing soda :- This removes the calcium and magnesium ions present in the water as insoluble calcium and magnesium trioxocarbonate (iv) respectively. The formula for washing soda is (Na2Co3).
Na2Co3 + CaSO4. —– CaCO3 + Na2SO4
Na2CO3 + MgSO4 —– MgCO3 + NaSO4
(c) Addition of caustic soda:- The caustic soda removes the calcium and magnesium ions from the water as insoluble calcium and magnesium hydroxides respectively.
2NaOH + CaSO4—- Ca(OH)2 + Na2SO4.
2NaOH + MgSO4—- Mg(OH)2 + NaSO4.
(d) Permutit method :- This method is used for softening water on a large scale, it is an ion-exchange resin. Permutit is a complex substance of hydrated sodium aluminum trioxosilicate(iv) commonly known as Sodiumzeolite. When hardwater is passed through the resin, the sodium ions will go into solution while the unwanted Calcium and magnesium ions take their place in the complex salt.
ADVANTAGES OF HARD WATER
(I) Hard water tastes better than soft water, because of the dissolved minerals in it.
(ii) The calcium salts present in hard water help to build strong bones and teeth.
(III) Hardwater helps animals like snails and crabs to make their shells.
(iv) Hard water can be supplied in leadpipes as it doesn’t dissolve lead but soft water dissolves lead which cause lead poisoning.
DISADVANTAGES OF HARDWATER
(I) It causes wastage of soap because it requires a lot of soap before it can form lather.
(II) It causes furring of kettles and boilers.
(III) The insoluble scum formed during washing with Hardwater causes dulling of white fibres.
(IV) Hard water cannot be used in dyeing or tanning as the salts in it interfere withheld mode of action of these processes.
ASSIGNMENT
- Permanent hardness of water is caused by ____ & _____
- Mention 4 ways of removing permanent hardness of water.
- State 3 advantages and disadvantages each of hardwater.
Tue, 9th June 2020
LESSON FOUR
Good day everyone, you are welcome to today’s class, we will be looking at the scheme of work for 3rd term together and any note being given to you here should be written in your CHEMISTRY note and to be submitted on the resumption day.
SCHEME OF WORK FOR 3RD TERM SS1
Week 1-2 Water
Week 3-5 Carbon and its compounds
Week 6-7 Introduction to volumetric analysis
Week 8 Calculations based on gas laws.
Week 9 Calculations based on pH scales.
WATER
Please listen to the by by voice notes very well for you to understand the notes that will follow.
WATER
Water is the most abundant substance in plant and animal tissues and as well as he world around us. It accounts for about 70% of he human body and over 80% of the Earth’s surface is covered by water.
TYPES OF WATER
There are two types of water, they are :-
Natural water and
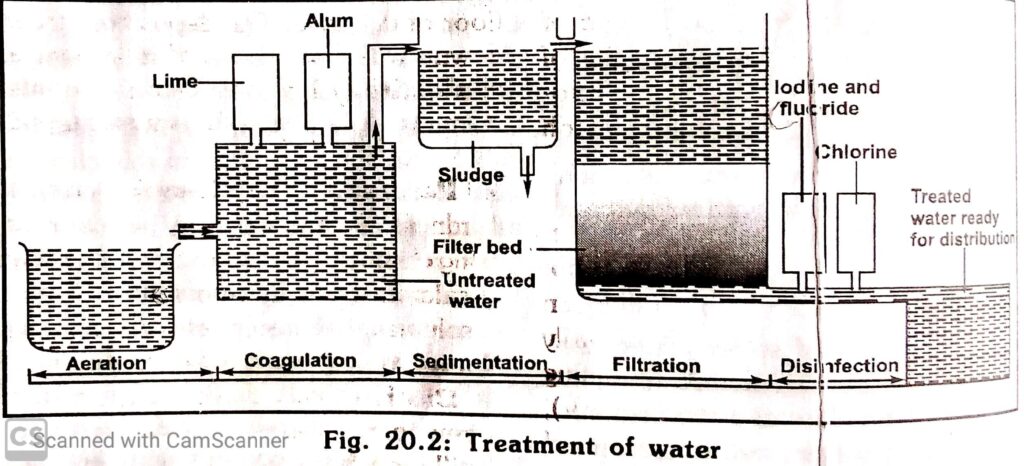
Treated water. 1. Natural water include rain water, spring water, well water, river water and sea water, among these, rain water is the purest form of natural water. 2. Treated water include distilled water, pipe- borne water and chlorinated water, and they are usually prepared for special purposes. TREATMENTAND SUPPLY OF WATER. Water for domestic use is prepared in a water- treatment plant by various methods which include:-. 1. Aeration:- untreated water from he source like rivers, lakes etc is aerated to remove volatile chemicals like hydrogen sulphide, carbon (IV) oxide and various odour from the water . 2. Coagulation:- This is also known as flocculation and it is when chemicals like potash alum (KAl(SO4)2) or sodium aluminate (III) (NaAlO2) are added to cause coagulation, i.e impurities clump together to form big particles of dirt which settle down rapidly.
Sedimentation:- This is the settling down of impurities to form a big particles underneath or below the water .
Filteration:- The water is passed through a filter bed to remove the remaining fine particles of dirt.
Disinfection:- The water is treated with chemicals like chlorine to kill germs, other useful chemicals like iodine and fluorine may be added in the correct amount as food supplement to prevent goitre and tooth decay.
The treated water is now clear and free from germs is stored in a reservoir and distributed to towns and cities through underground pipes for domestic and industrial uses . HARDNESS OF WATER Hardness of water is a property that describes the type of water that doesn’t lather readily with soap, and this type of water is known as hard water. Hardness of water is due to the presence of dissolved: (i)Calcium tetraoxosulphate (vi) (CaSO4) (ii)Magnesium tetraoxosulphate (vi) (MgSO4) (iii)Calcium hydrogen trioxocarbonate (iv) Ca(HCO3)2 Soap is the sodium or potassium salt of an organic acid, so when soap is added to hard water, the dissolved salt in the water will react with he soap to form insoluble salt of calcium or magnesium which form an unpleasant scum that sticks to clothes and it is difficult to rinse away.
TYPES OF HARDNESS OF WATER. There are 2 types of hardness of water. 1. Temporary hardness and 2. Permanent hardness. 1. TEMPORARY HARDNESS :- This type of hardness is caused by the presence of dissolved calcium hydrogen trioxocarbonate (iv), Ca(HCO3)2 which decomposes on heating. REMOVAL OF TEMPORARY HARDNESS. 1. Boiling :- Boiling decomposes the Ca(HCO3)2 present in the water to form insoluble CaCO3. E.g Ca(HCO3)2 boil CaCO3 +H2O +CO2. The removal of the calcium ion will make the soap added to water to form lather. (ii) Addition of slaked lime:- Temporary hardness can also be removed by adding a calculated amount of slaked lime Ca(OH)2. CaCO3 is precipitated and can be filtered off. E g Ca(HCO3)2 + Ca(OH)2 ____ 2CaCO3 +2H2O. If excess slaked lime is added, it makes the water to be permanently hard.
EFFECT OF TEMPORARY HARDNESS
1. Furring of kettles and boilers :- Kettle or boiler used for boiling temporarily hard water will have the inner surface become coated with a layer of Calcium trioxocarbonate (iv) from the decomposition of calcium hydrogen trioxocarbonate (iv). (ii) Stalagmites and stalactites :- These pillars of almost pure calcium trioxocarbonate (iv) are made by temporarily hard water dripping down from the roof to the floor of the hot cave. The deposition growing downwards from the roof of the cave is known as stalactite while he one growing upwards from the floor is known as stalagmite.
Assignment
- What is hard water?
- State the various methods used in the purification of water.
- State 2 effects of temporary hardness of water
- State 2ways by which you can remove temporary hardness of water.
LESSON THREE
Date: – Tue,2nd June 2020
Topic:- Chemical combination (contd)
Good day everyone and how has your day been?
Listen to the voice note below carefully for proper explanation.
Good day students, hope all is well with you over there? May God’s grace be sufficient for us in Jesus name.
Today we will be looking at the topic :-
CHEMICAL COMBINATION
Watch the below video for proper explanation.
Now answer the following questions after watching the above video.
- Write briefly on the following, giving an illustrated example of each:- (a) Electrovalent combination (b) Covalent combination
- What are the bond types present in each of the following compounds? (i) Carbon (IV) oxide (ii) Calcium oxide. (iii) Methane. (iv) Sodium chloride.
Hello everyone, hope you are all staying safe and hope you are reading at home as well? You are welcome to today’s class and we will be looking at one of the interesting topics in chemistry. Our topic today is separation techniques.
SEPARATION TECHNIQUES
Introduction





DISTILLATION
Distillation is used to recover solvent form solution, In other words it is a price of vaporizing a liquid and then condensing the vapour.
EVAPORATION
This is a method of separating a dissolved solute from a solution by heating the mixture to dryness so that the liquid portion with a lower vapour pressure than the solid is removed from the solid portion. E.g salt solution.
Fractional distillation is used to separate a mixture of two or more mixed liquids with close boiling points into its component fractions.
DECANTATION
This is a method used to separate a mixture of liquid and denser solid particles which separate into two distinct layers on standing. The upper layer is carefully poured from he bottom solid portion into another container.
CHROMATOGRAPHY
This is a method of separating the constituents of a mixture by taking advantage of their different rates of movement in a solvent over an adsorbent medium. It can b used to separate mixture of colours, dyes, leaf pigments etc.
CENTRIFUGATION
This method is used to separate suspension into its component fractions e.g blood.
CRYSTALLIZATION
This is use to separate salts which decompose easily on heating from their solutions.
PRECIPITATION
This process uses the property of differences in solubilities of solids in different mixable liquids to brin out the solid.
CLASS WORK.
- State what separation method you would use for the following mixtures. (a) kerosene and water. (b)alcohol and water. (c) blood. (d)sand and iron fillings
- Give one industrial application of the following separation techniques. (a)evaporation. (b)filteration. (c) fractional distillation. (d) sieving